¶ Objective: Disease Eradication
🚀An 80X acceleration in medical progress by upgrading fda.gov and giving all patients the right to effortlessly participate in global automated decentralized clinical trials.
¶ Horrible Problems We Can Solve
👥 ~$43,000 cost per participant in traditional clinical trials
💸 ~$2.2 Billion cost to develop a new treatment falls on patients
☠️ 21,000 to 120,000 People more people die each decade due to suboptimal regulatory processes
🚫 85% of patients are not allowed to participate in clinical trials
💊 95% of rare diseases have NO FDA-approved treatments
🤒 17 years of suffering is endured from discovery of a new treatment until it reaches patients
⚛️ 166 billion potential treatments have not been tested
🌍 2 Billion people are suffering from uncured diseases
🧫 It's been 44 years since we last cured a disease
🙈 Negative Results are Never Published so we waste billions of dollars repeating failed drug trials.
📊 No Treatment Effectiveness Rankings - The existing system does not give us a ranked list of the most safe and effective treatments for a given condition.
🥫 No Data on Unpatentable Substances - We still know next to nothing about the long-term effects of the cocktail of over 330 chemical additives we consume.
🗓️ No Long-Term Outcome Data - Current trials often only last a few months, so we have no idea what the long-term positive or negative effects of a drug are!
🤒 People With Rare Disease are Severely Punished - It's impossible to recover a billion dollars of drug development from a small number of patients.
🔮 Pre-Determining Clinical Endpoints Requires Psychic Powers - Drug Developers to Have Psychic Powers Needed to Pre-Determine Very Specific Clinical Endpoints Before Collecting Data
🥸 Trials Often Aren't Representative of Real Patients - Clinical trials often exclude as much as 85% of patients so they don't tell us if the drug will work for the majority of patients.
¶ How To Fix It: Upgrade FDA.gov 🚀
With a small fraction of the FDA's $7 billion annual budget to upgrade FDA.gov, we can allow ANY patient ANYWHERE in the world to:
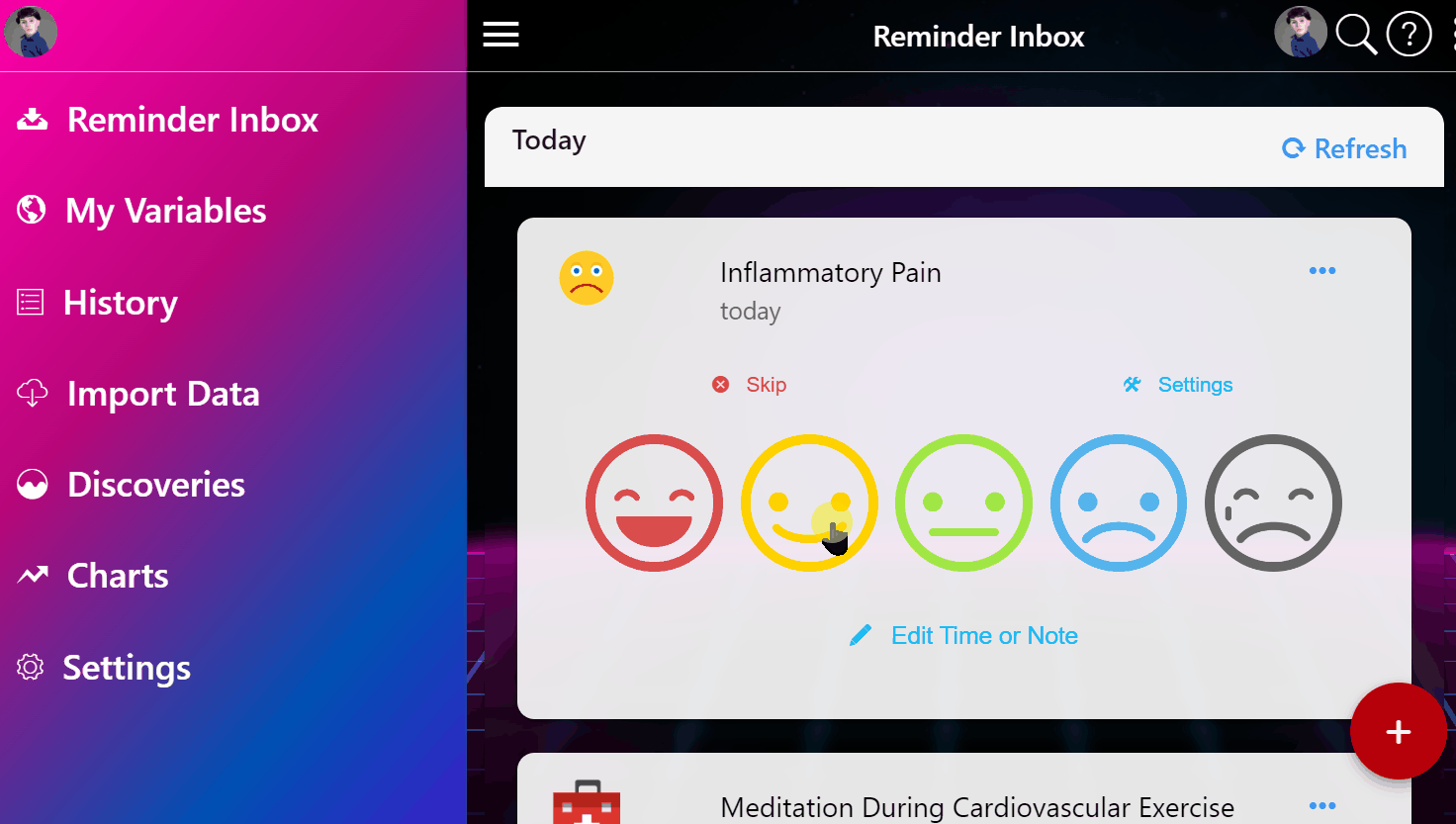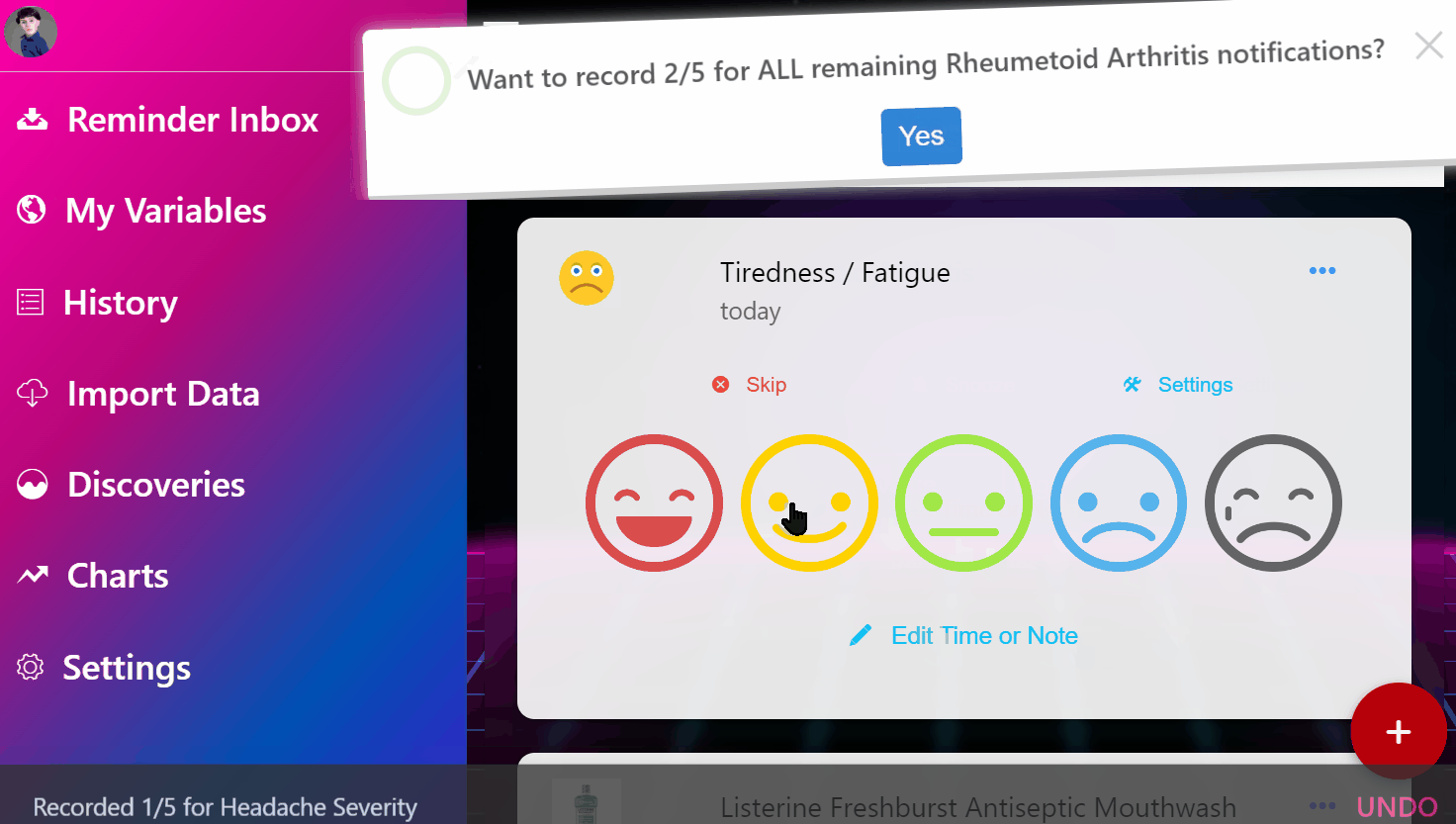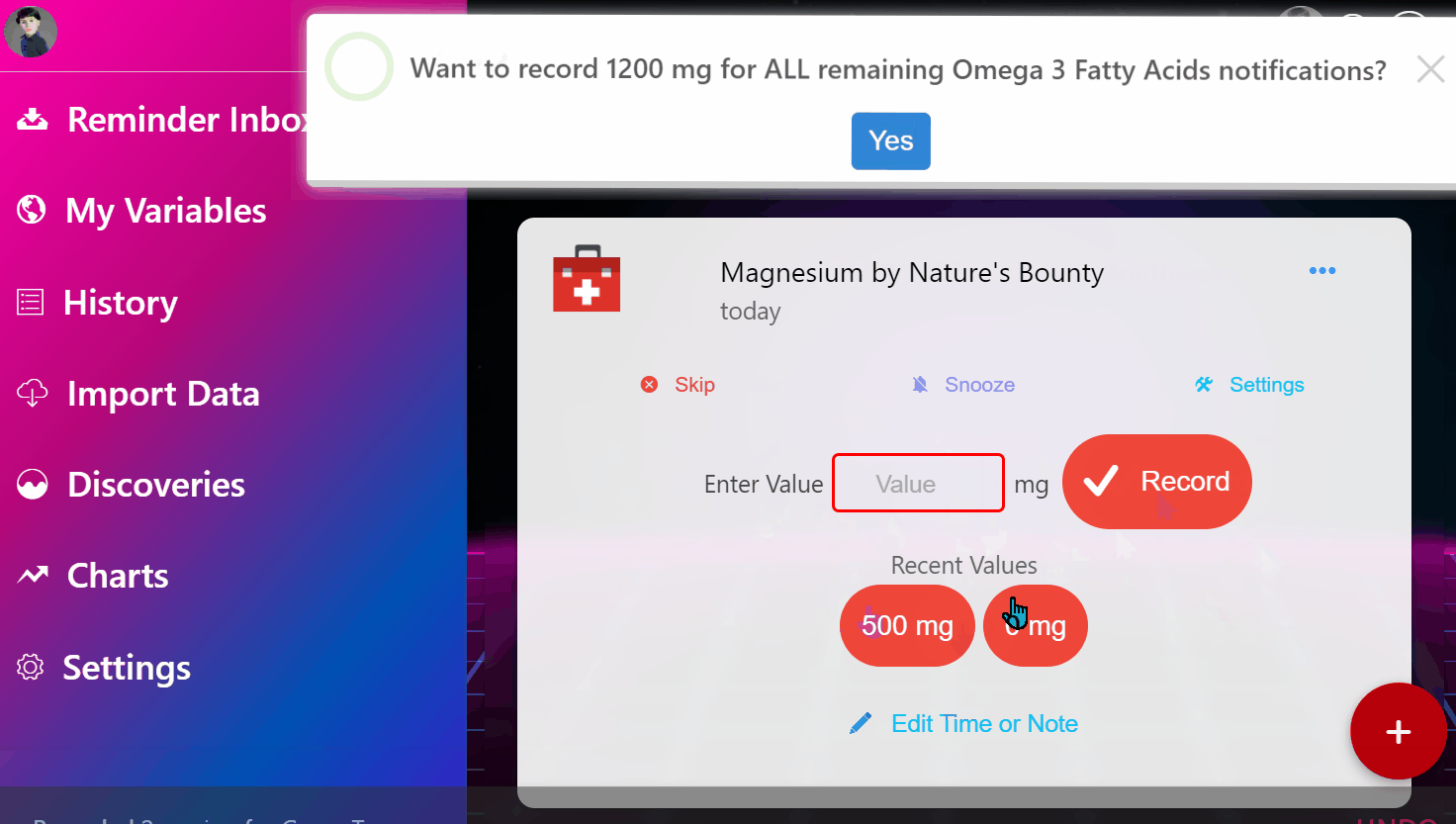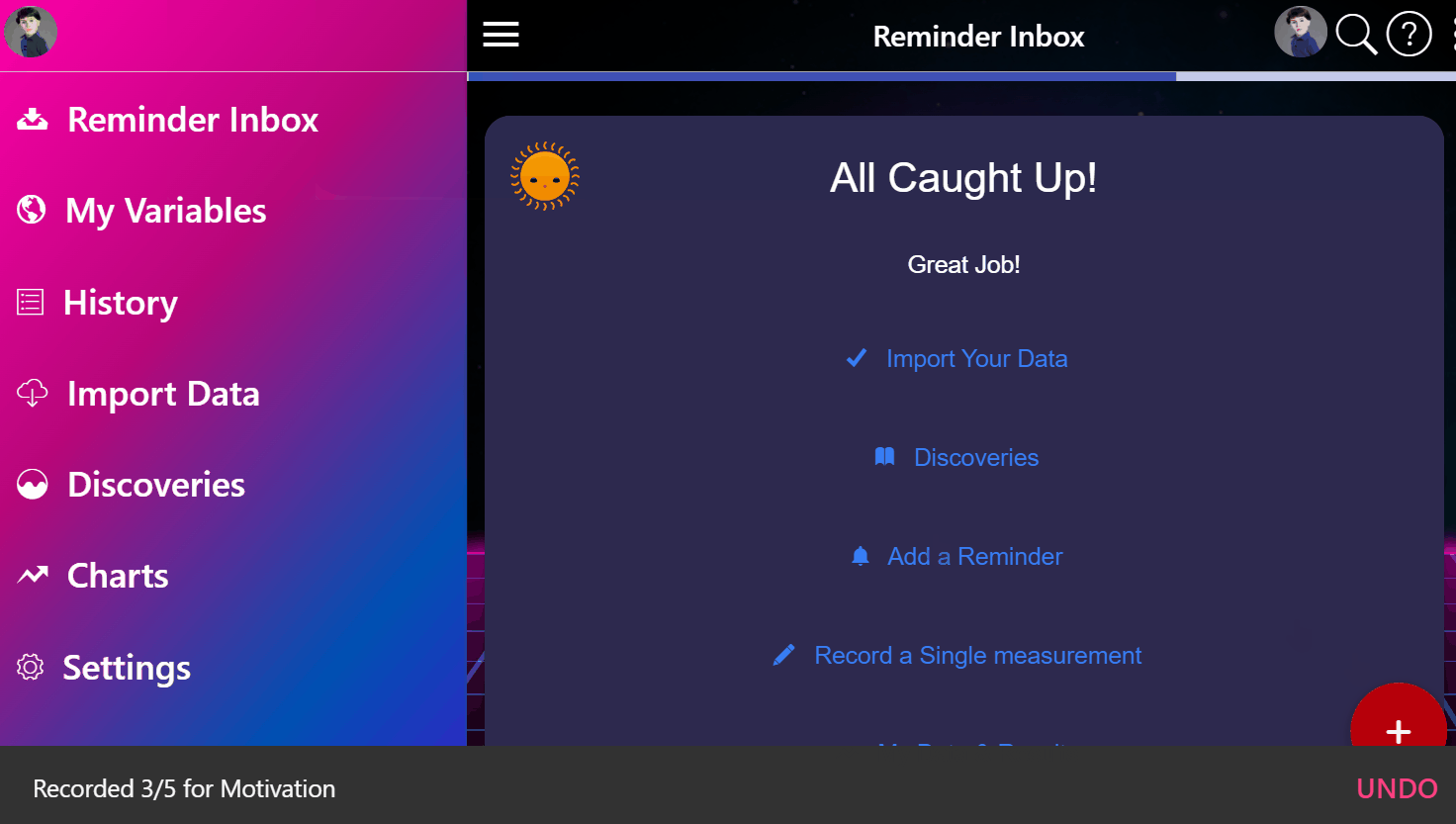
¶ 1. Enter Your Condition
Simply input the condition that's making you miserable.

¶ 2. View Ranked Treatments
See a ranked list of the most safe and effective treatments based on the entire universe of clinical and real-world data.
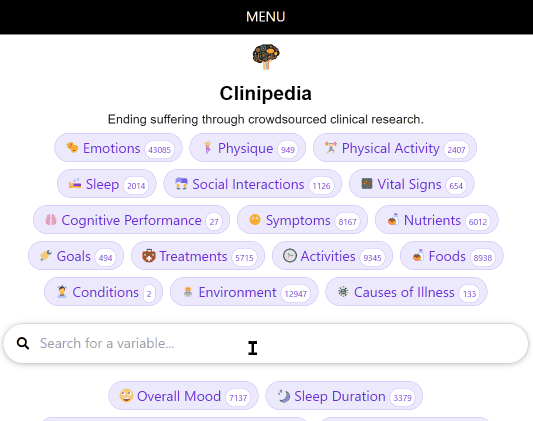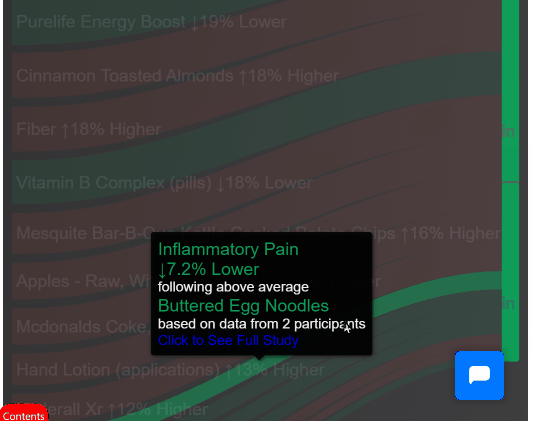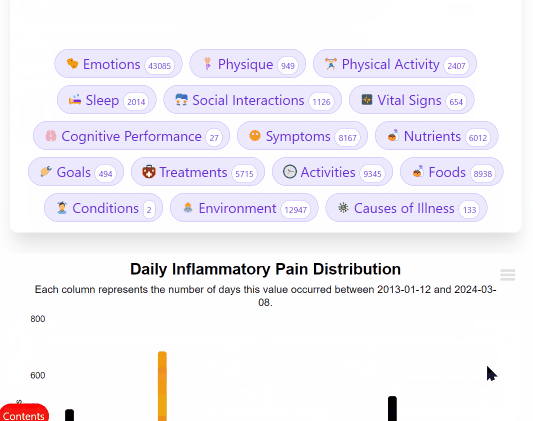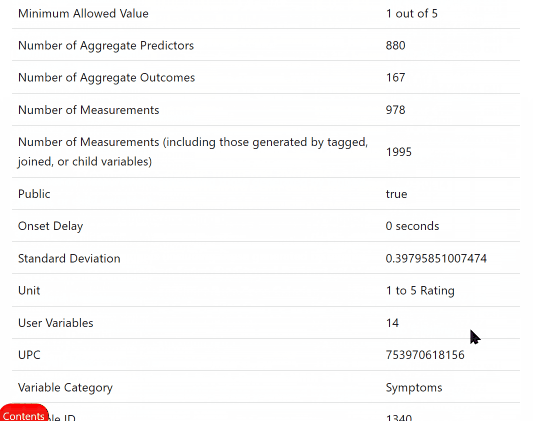
¶ 3. Join Clinical Trials from Home
Instantly enroll in decentralized trials for promising treatments that interest you.
¶ 4. Get Treatment Delivered
Get treatments delivered to your pharmacy or physician and schedule necessary lab tests automatically.
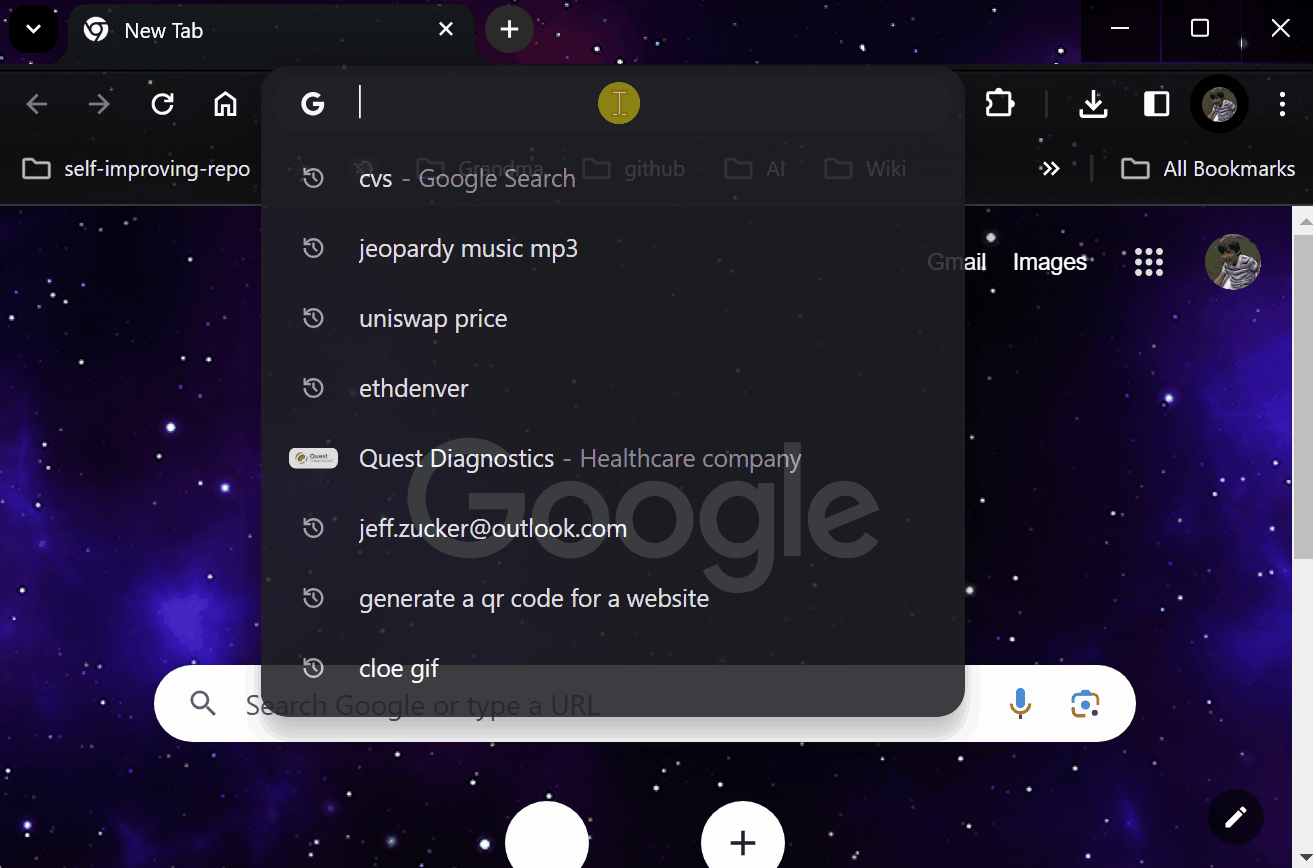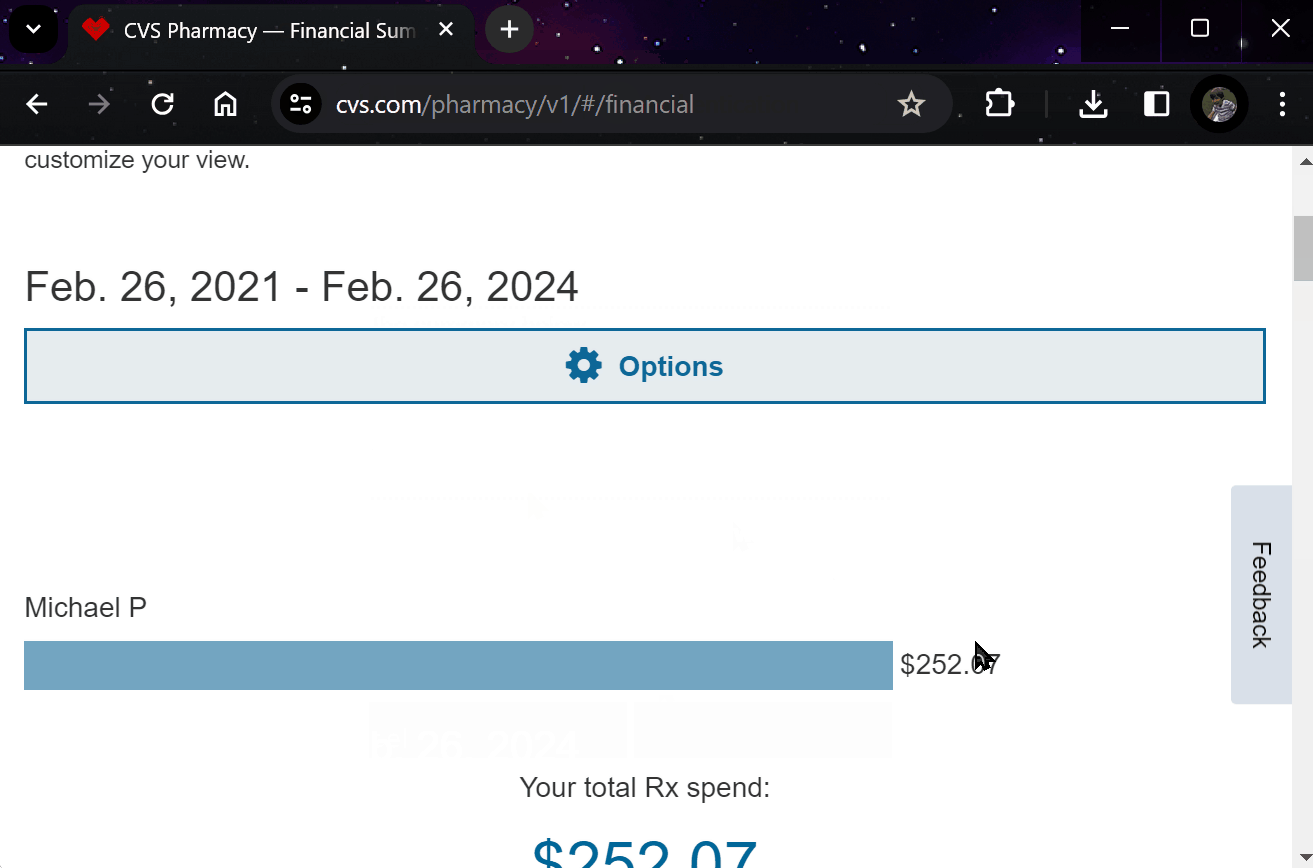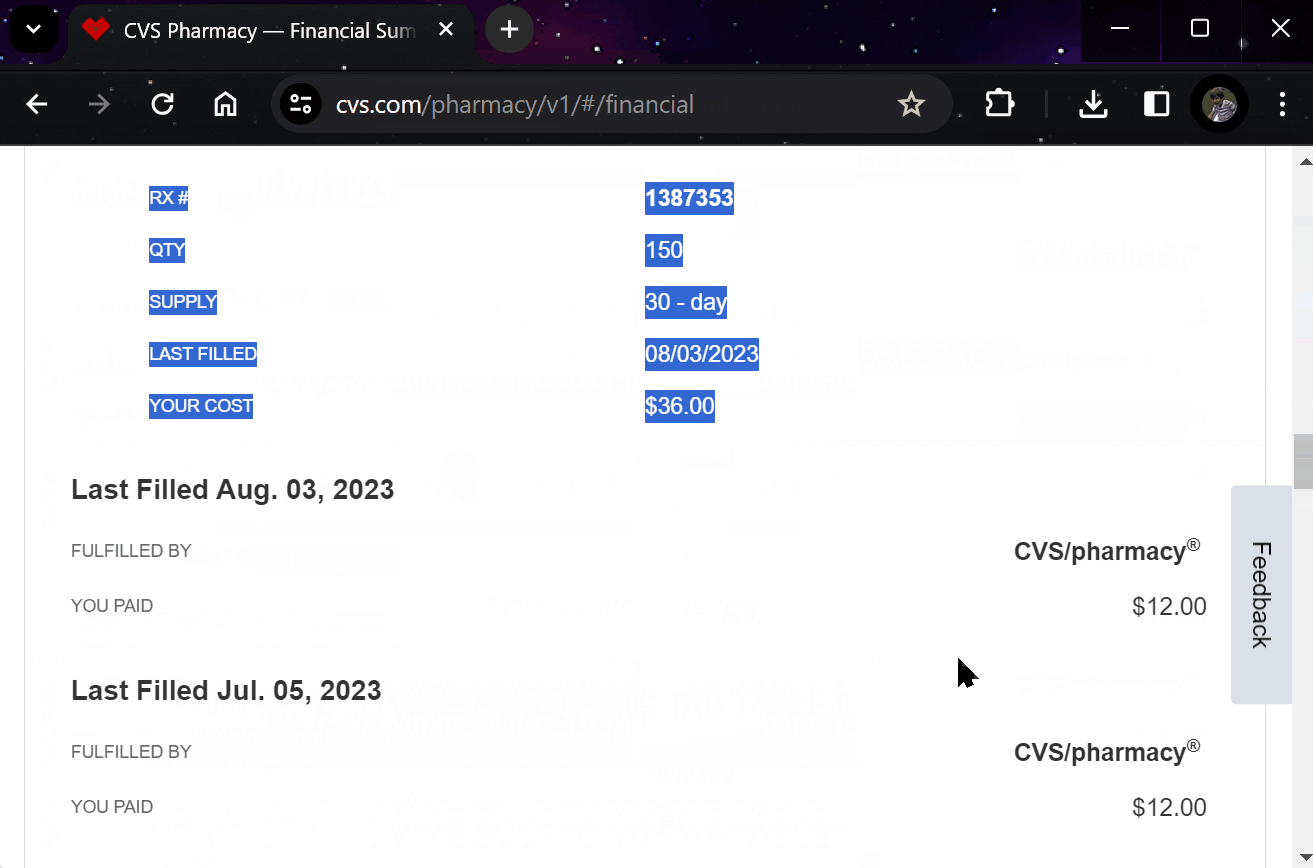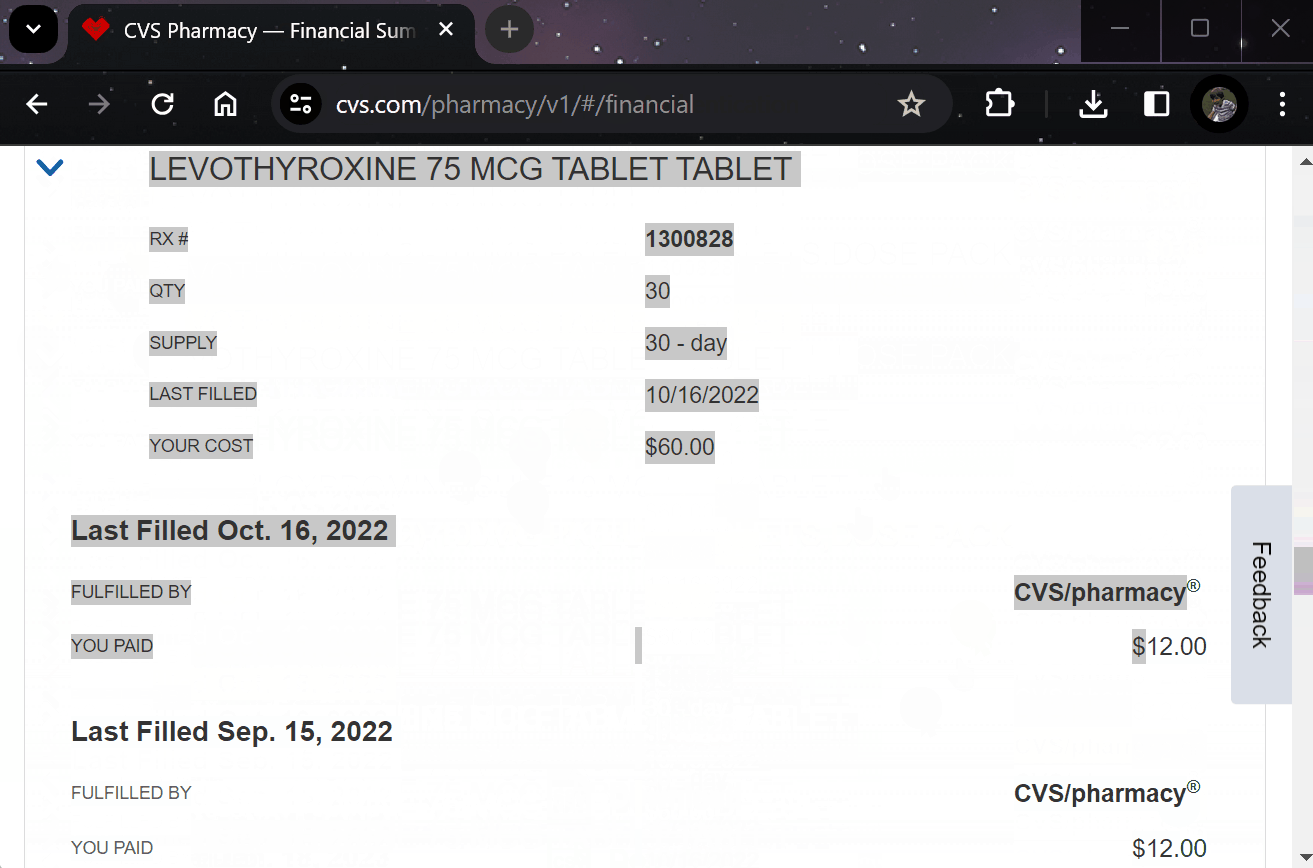
¶ 5. Effortlessly Report Outcomes
Report treatment effects easily through your preferred apps, EHR systems, or automated calls.
¶ 6. Continuously Improve Rankings
Your data helps improve treatment rankings and benefits future patients globally.
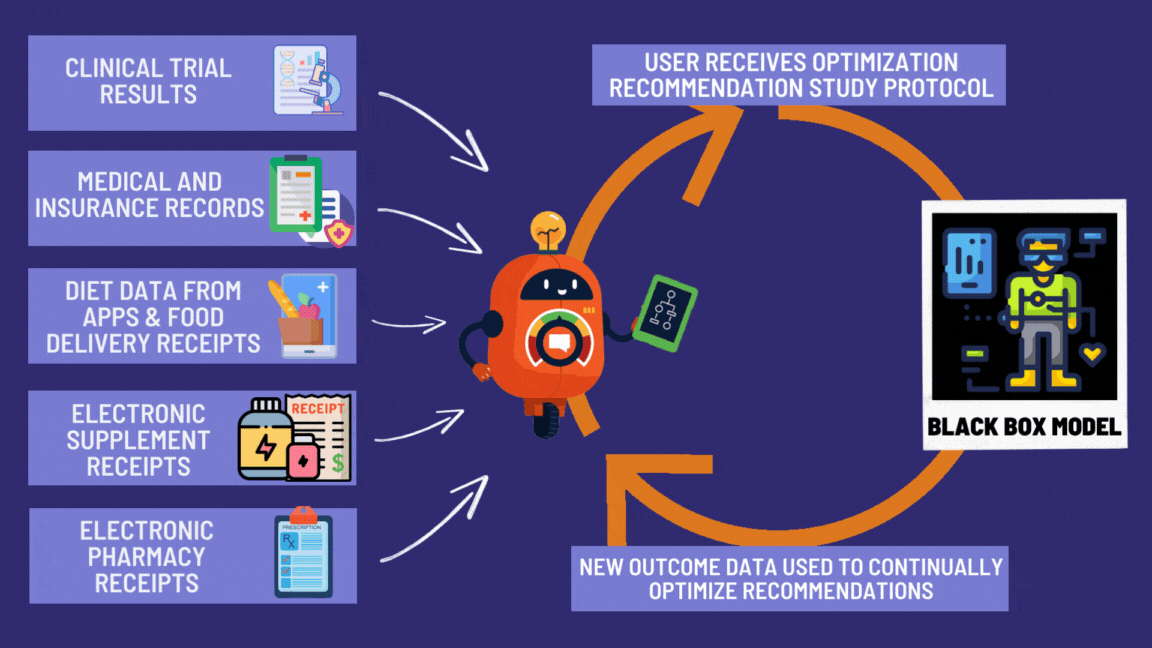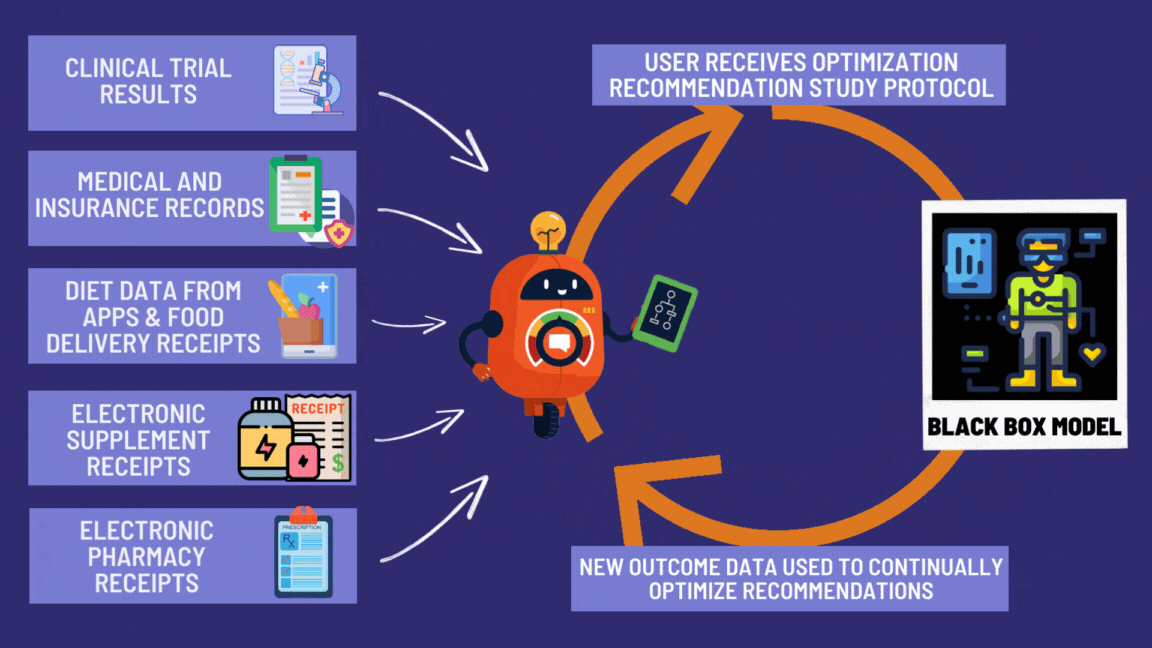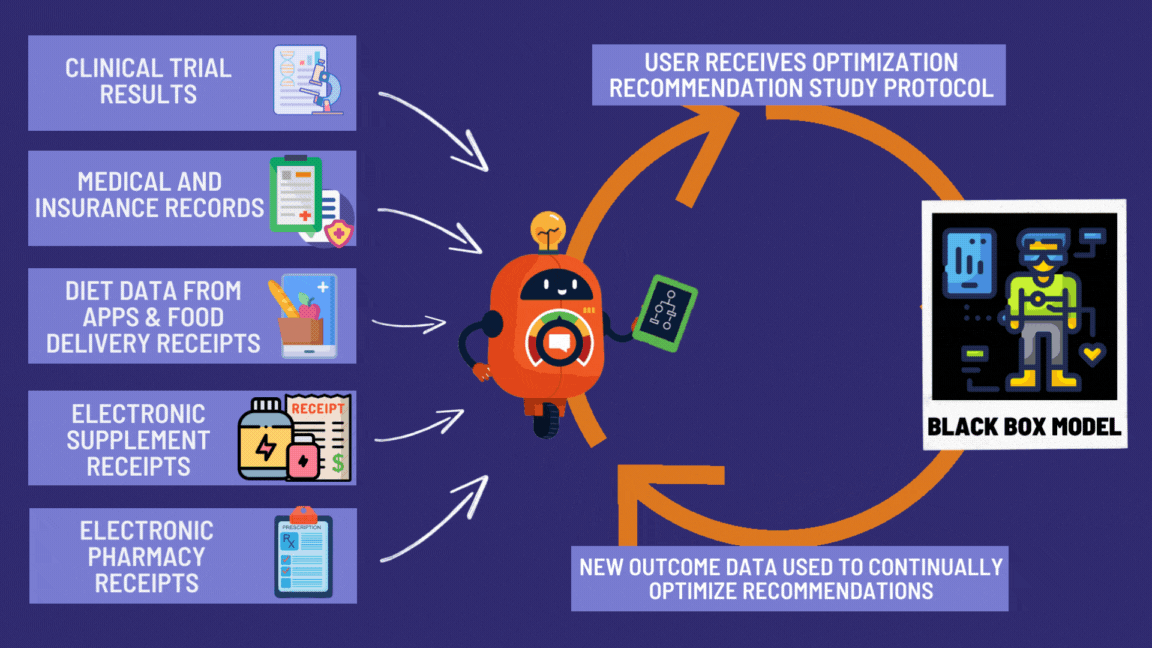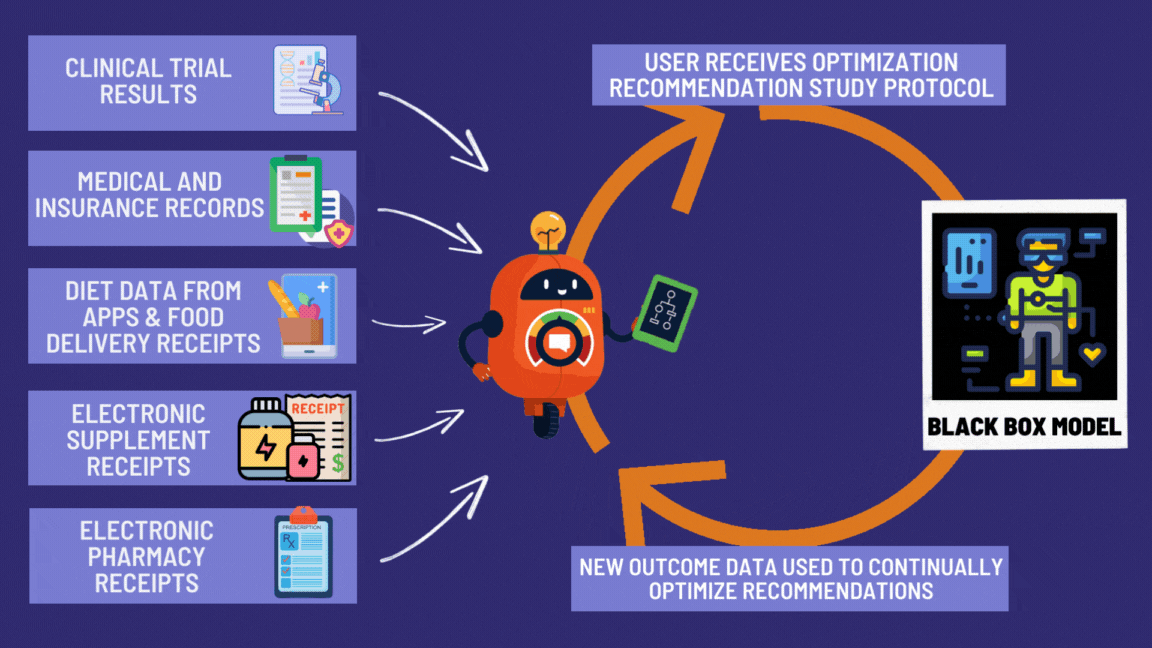
¶ Benefits of the Global Disease Eradication Act
Look at all these great features giving patients the right to effortlessly participate in decentralized clinical trials! 🚀
| Category | FDA v1 | FDA v2 |
|---|---|---|
| ⏱️ Years Until Patients Can Access Treatment | 17 years | ➡️ 2 years |
| 💰 Cost of Clinical Trials | ~$57M | ➡️ $2M |
| 👥 Percent of Patients Able to Join Trials | 15% | ➡️ 100% |
¶ 👀Look at those savings! 🤑
By decentralizing and automating clinical research, we could reduce the cost of new treatments by 95%!
| Cost Item | Current Cost | New Cost | Savings |
|---|---|---|---|
| 💾 Data Management | $198,014 | $10,000 | 94.9% |
| ✅ Cost Per IRB Approvals | $324,081 | $5,000 | 98.5% |
| 📝 IRB Amendments | $6,347 | $0 | 100.0% |
| 🔍 SDV | $1,486,250 | $25,000 | 98.3% |
| 🤝 Patient Recruitment | $805,785 | $15,000 | 98.1% |
| 🎯 Patient Retention | $76,879 | $20,000 | 74.0% |
| 👨⚕️ Nurse/Research Associate | $2,379,605 | $150,000 | 93.7% |
| 👩⚕️ Physician | $1,966,621 | $100,000 | 94.9% |
| 🏥 Clinical Procedure Total | $5,937,819 | $1,000,000 | 83.2% |
| 🧪 Laboratory | $2,325,922 | $500,000 | 78.5% |
| 🏢 Site Recruitment | $849,158 | $0 | 100.0% |
| 🏗️ Site Retention | $4,461,322 | $0 | 100.0% |
| 👥 Administrative Staff | $7,229,968 | $100,000 | 98.6% |
| 📊 Site Monitoring | $4,456,717 | $0 | 100.0% |
| 🏢 Site Overhead | $7,386,816 | $0 | 100.0% |
| 📎 All Other | $17,096,703 | $100,000 | 99.4% |
| TOTAL SAVINGS 🎉 | $56,988,007 | $2,025,000 | 95.7% |
Cost analysis based on data from: Examination of Clinical Trial Costs and Barriers for Drug Development by the U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation (ASPE).
To further reduce patient costs, new financial models like the Decentralized Institutes of Health are being proposed to fund trial participation through innovative means.
¶ Platform Architecture

¶ Check Out These Great Features! 👀
See all the wonders we could enjoy with the passage of the Disease Eradication Act!
¶ Your Personal FDAi Agent
Help us give everyone a free superintelligent doctor
¶ Your Digital Twin Safe
Securely store and control your health data

¶ Outcome Labels
See how treatments affect specific health outcomes
¶ Automated Data Collection
Import data from wearables and apps.

¶ Automated Analysis
Analyze your health data to identify patterns and potential root causes of your condition.
¶ Real-Time Decision Support
Get personalized insights and recommendations based on your real-time health data analysis.
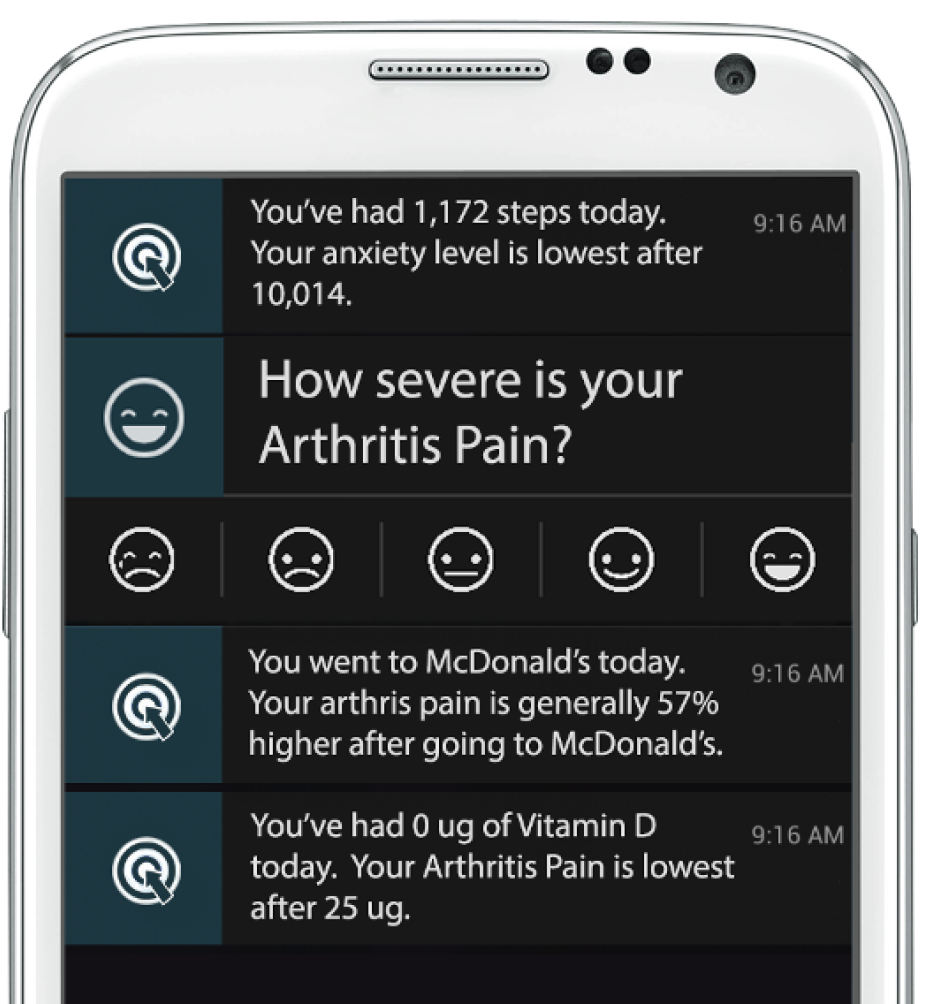
¶ Create Your Own Studies
Anyone can create a study, become a prestigious scientist, get a link, and invite all their friends to join!
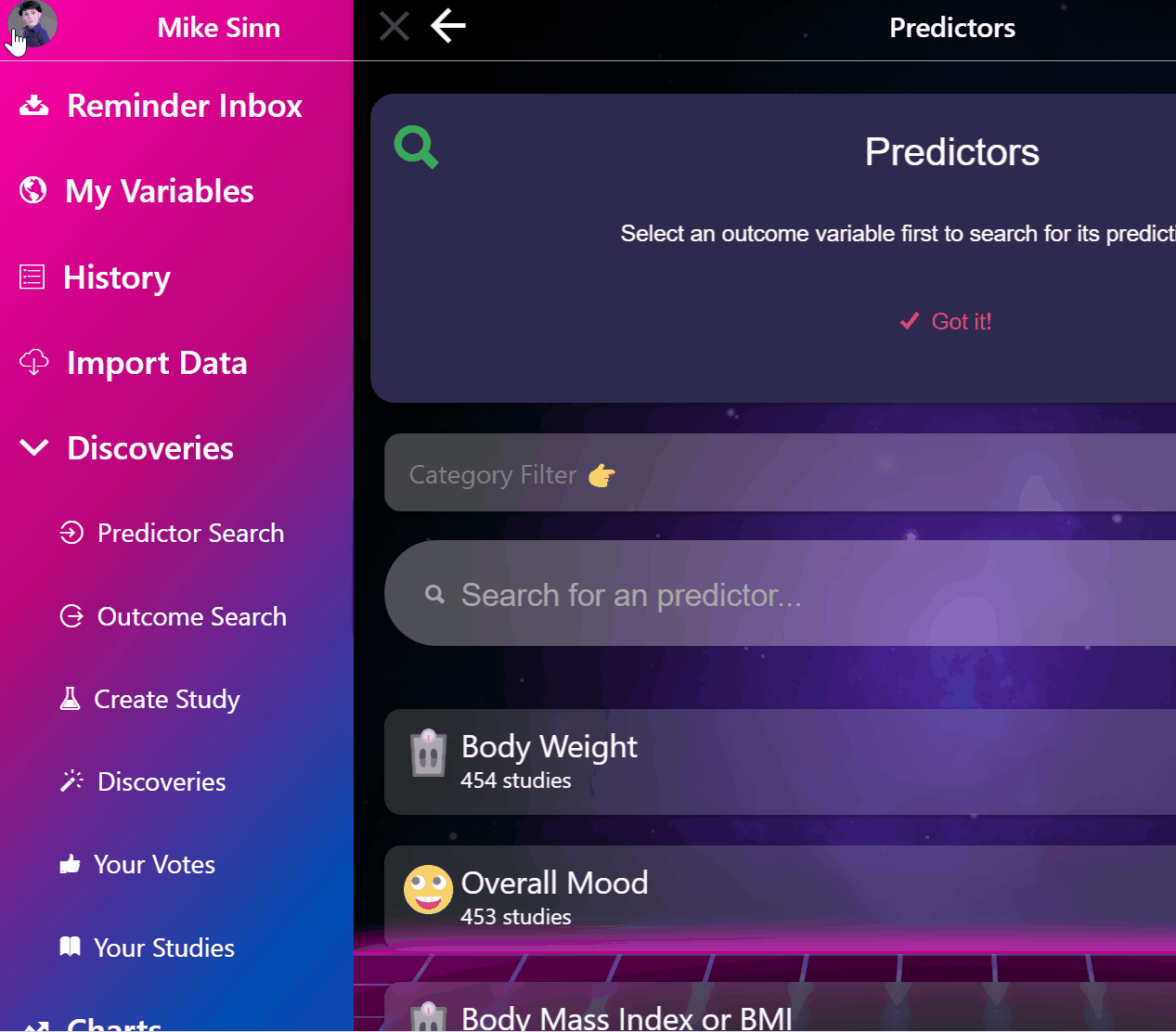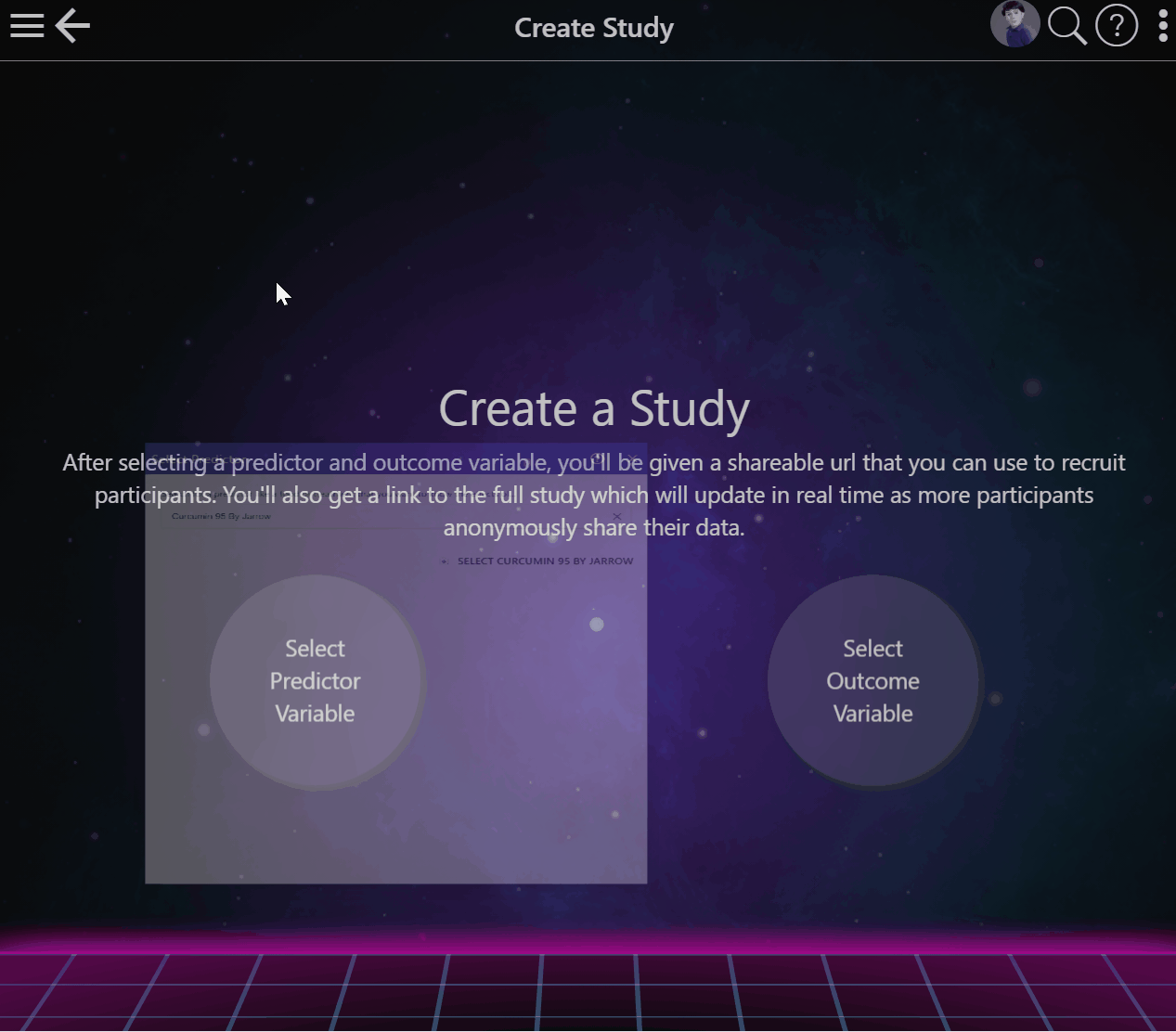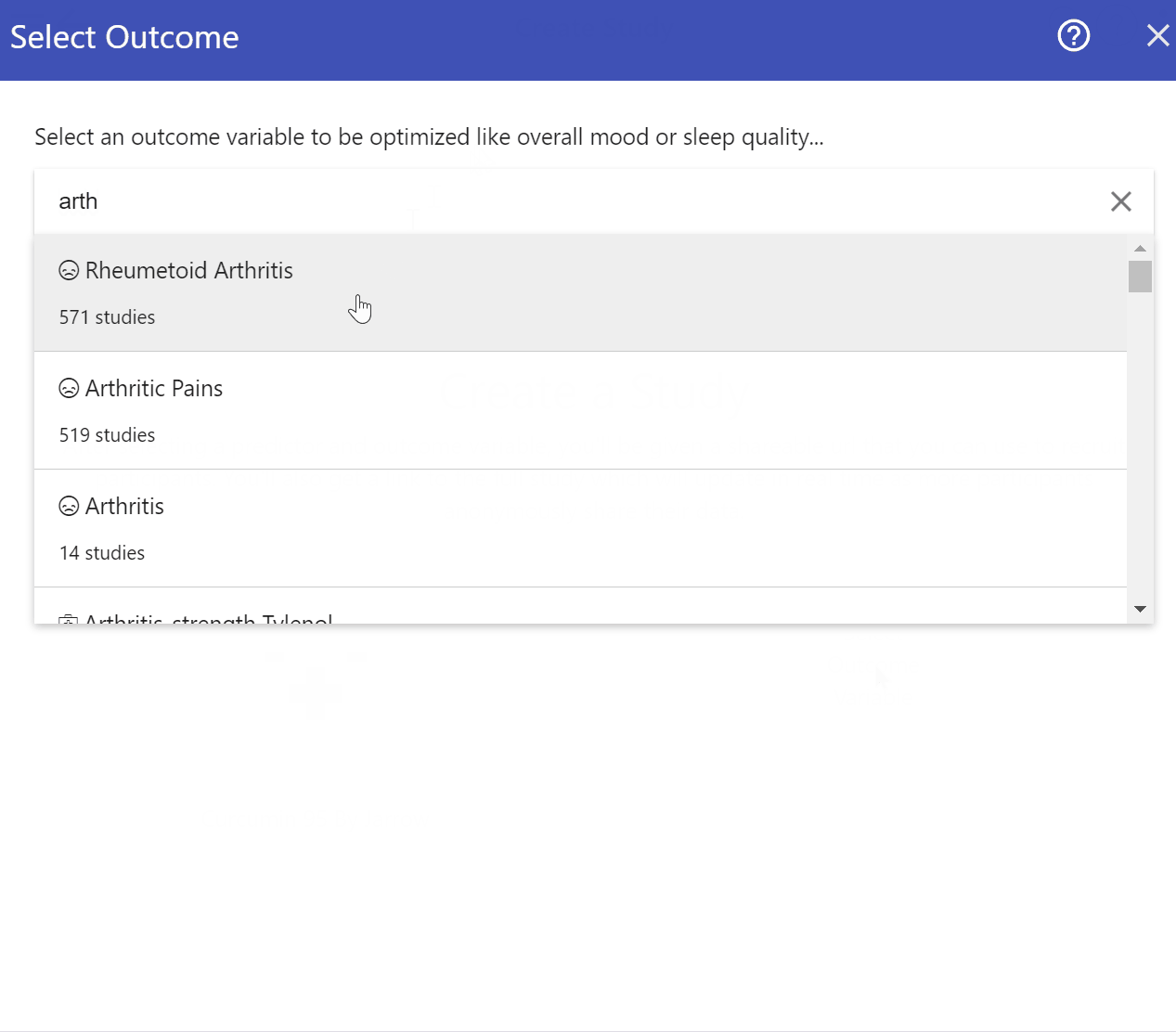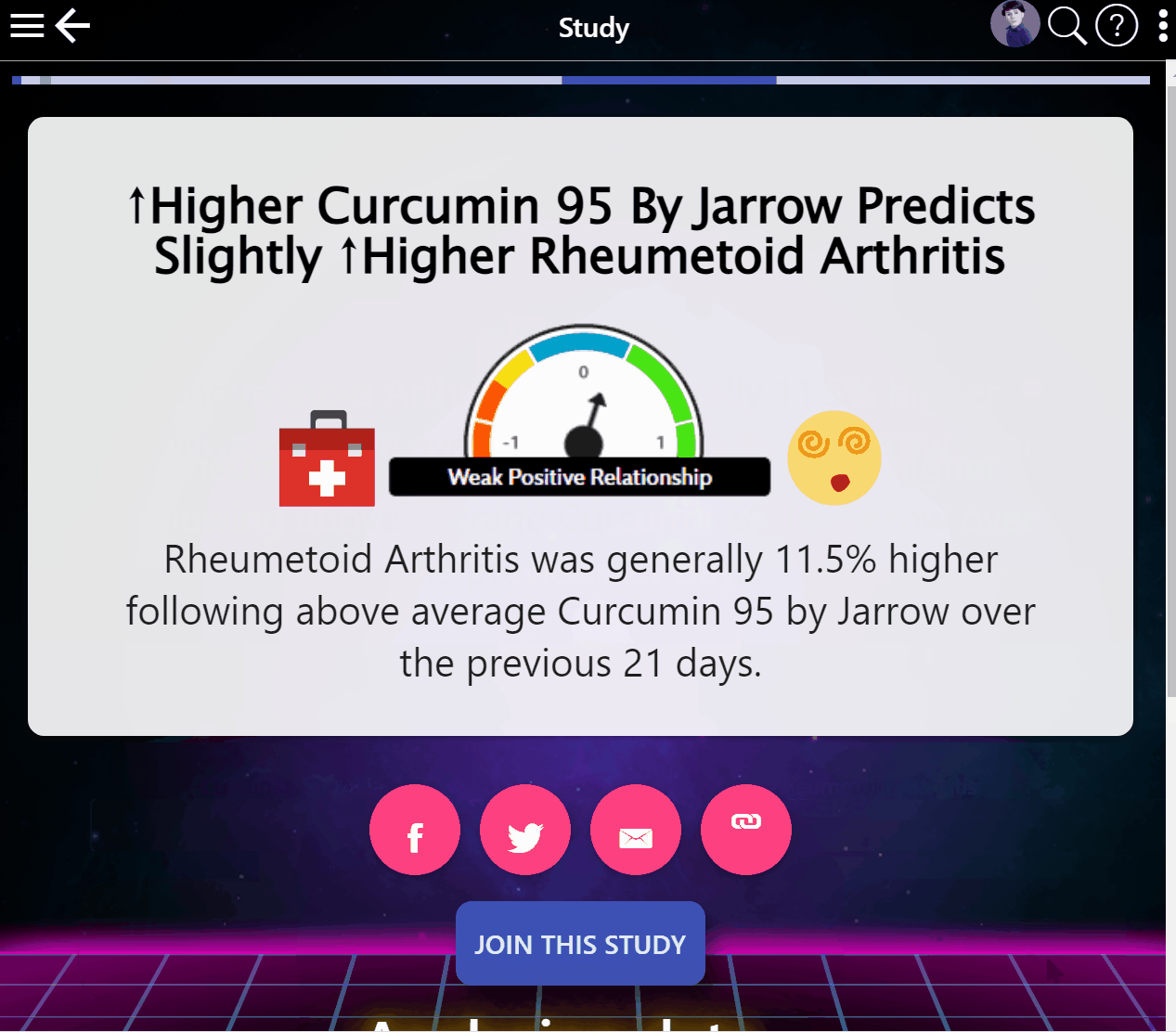
¶ Global Scale Studies
Studies are published in a Wikipedia for clinical research based on everyone's data, listing the likely effects of every food and drug.

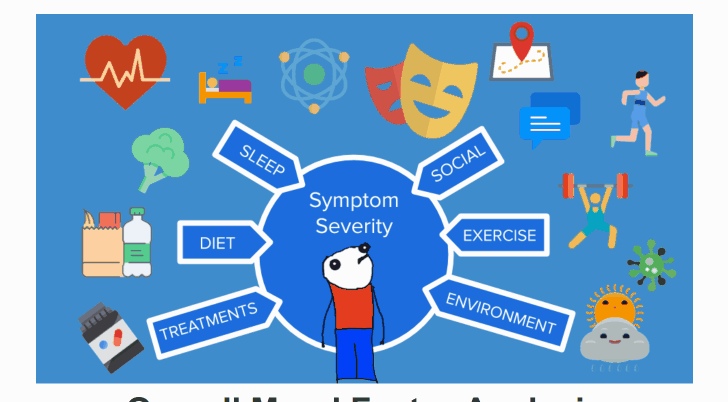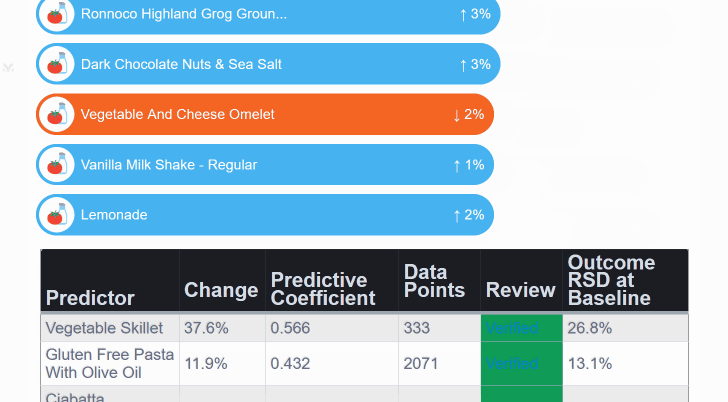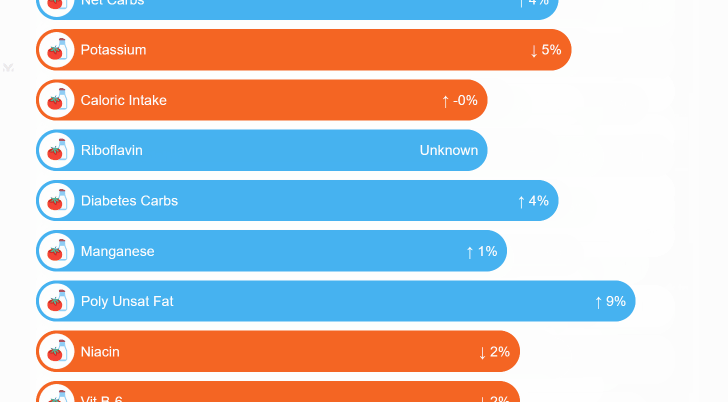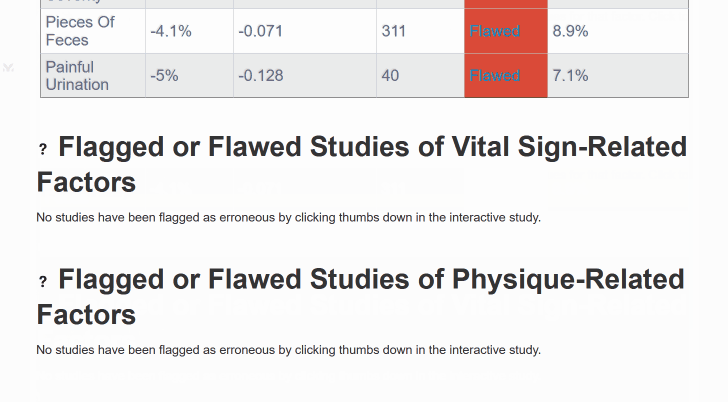
¶ Mega-Studies on Specific Conditions
Look up your condition and see how different foods, drugs and supplements tend to improve or worsen your condition.
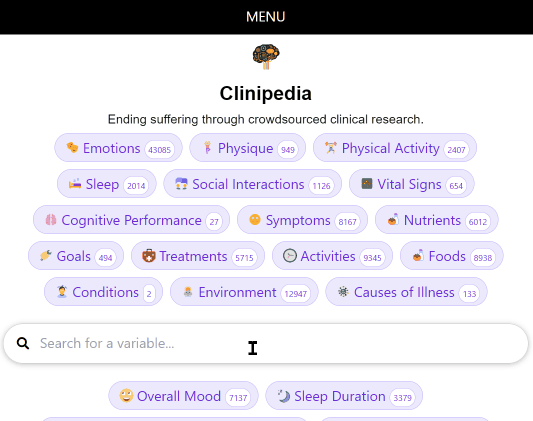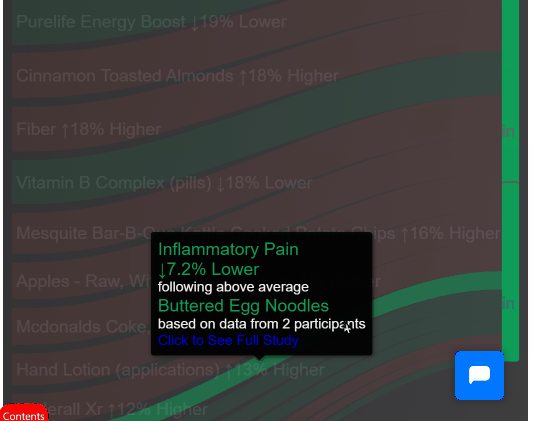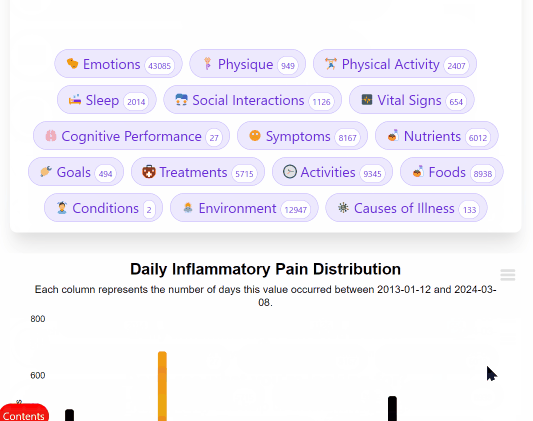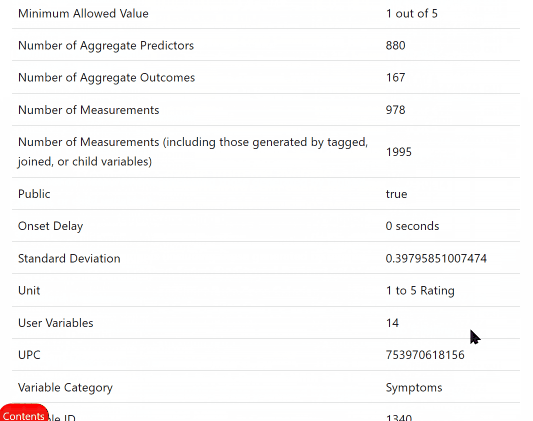
¶ The Disease Eradication Act
Show your support to give people suffering access to the most promising treatments
¶ Stay Up to Date
Join our newsletter to receive updates on clinical trials, research breakthroughs, and ways to get involved.
We'll keep you informed about the latest developments in decentralized clinical research.
- dFDA
- Studies
- Digital Twin Safe
- FDAi
- Disease Eradication Act
- 50/50 Health Savings Sharing Program
- Report Bug
- Request Feature
- GitHub
¶ Citations
-
Claim: ~$43,000 cost per participant in traditional clinical trials.
"Median per‑patient cost for a phase‑3 drug trial in 2024 exceeded $43,000"
-
Claim: ~$2.2 Billion cost to develop a new treatment falls on patients.
"The average cost for a Big Pharma to develop a drug in 2024 was $2.23 billion, up from $2.12 billion the year before."
-
Claim: 21,000 to 120,000 more people die each decade due to suboptimal regulatory processes.
"The annual number of lives lost in the United States because of the drug lag is in the same range as the number of lives lost annually in automobile accidents. The low estimate is 21,000 lives lost per decade, about one-fifth the number of people killed in automobile accidents during the same period. The high estimate is 210,000 lives lost per decade, about double the number of people killed in automobile accidents."
-
Claim: 85% of patients are not allowed to participate in clinical trials.
"Up to 85 percent of patients are excluded from participating in pivotal trials, limiting the generalizability of findings to real-world patient populations."
-
Claim: 95% of rare diseases have NO FDA-approved treatments.
"an estimated 95 percent of rare diseases lack a single FDA-approved treatment"
-
Claim: 17 years of suffering is endured from discovery of a new treatment until it reaches patients.
"New, effective treatments take an average of 17 years to transition from scientific discovery to clinical practice"
-
Claim: 166 billion potential treatments have not been tested.
"Sixteen years on, he has amassed the largest database of small molecules in the world, a gigantic virtual collection of 166 billion compounds."
-- How my race to find a drug for my daughter is helping others
-
Claim: 2 Billion people are suffering from uncured diseases.
"Incidence of acute sequelae were predominantly infectious diseases and short-term injuries, with over 2 billion cases of upper respiratory infections and diarrhoeal disease episodes in 2013..."
-
Claim: It's been 44 years since we last cured a disease.
"In 1980 WHO declared smallpox eradicated – the only infectious disease to achieve this distinction."
-
Claim: Negative Results are Never Published.
"Half of US clinical trials go unpublished"
-
Claim: We still know next to nothing about the long-term effects of the cocktail of over 330 chemical additives we consume.
"The cocktail effect of all these additives has also never been investigated. They may generate a health effect that doesn't happen when the additives are used individually."
-
Claim: It's impossible to recover a billion dollars of drug development from a small number of patients.
"The average cost of drug development from 2009 to 2018 was $985 million"
-
Claim: Cost analysis based on data from HHS report.
"This study models the decision-making process for a drug sponsor as a stylized decision tree that looks at the process for formulating a clinical trial from the point of view of an expected-revenue-maximizing sponsor in the face of uncertainty (or risk)."
-- Examination of Clinical Trial Costs and Barriers for Drug Development
-
Claim: The dFDA platform can accelerate trial timelines to 2 years.
"24 months: Universal enrolment guarantee active; subsidies flowing."
-
Claim: The dFDA platform can reduce trial costs to ~$2M.
"The total cost of the UK Oxford RECOVERY trial was £2.1 million (~$2.7M)... This is nearly 80 times more cost-efficient than traditional clinical trials"
-
Claim: The dFDA platform can enable 100% patient participation.
"any U.S. resident who requests an investigational intervention shall be guaranteed enrolment—remotely if necessary—in at least one pragmatic, decentralized trial arm evaluating that intervention"
